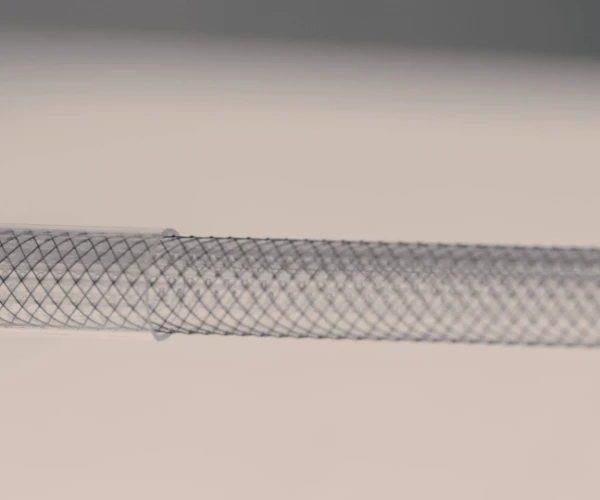
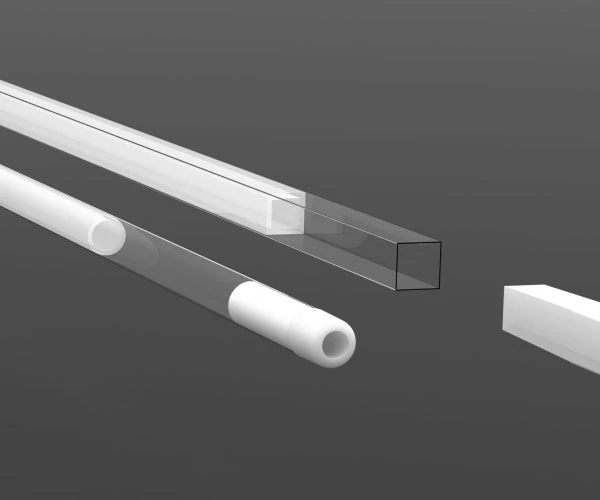
Finished catheters and medical devices
ENKI positions itself as a contract manufacturer for the assembly of complete medical devices in an ISO 7 cleanroom, ensuring the highest standards of quality and reliability.

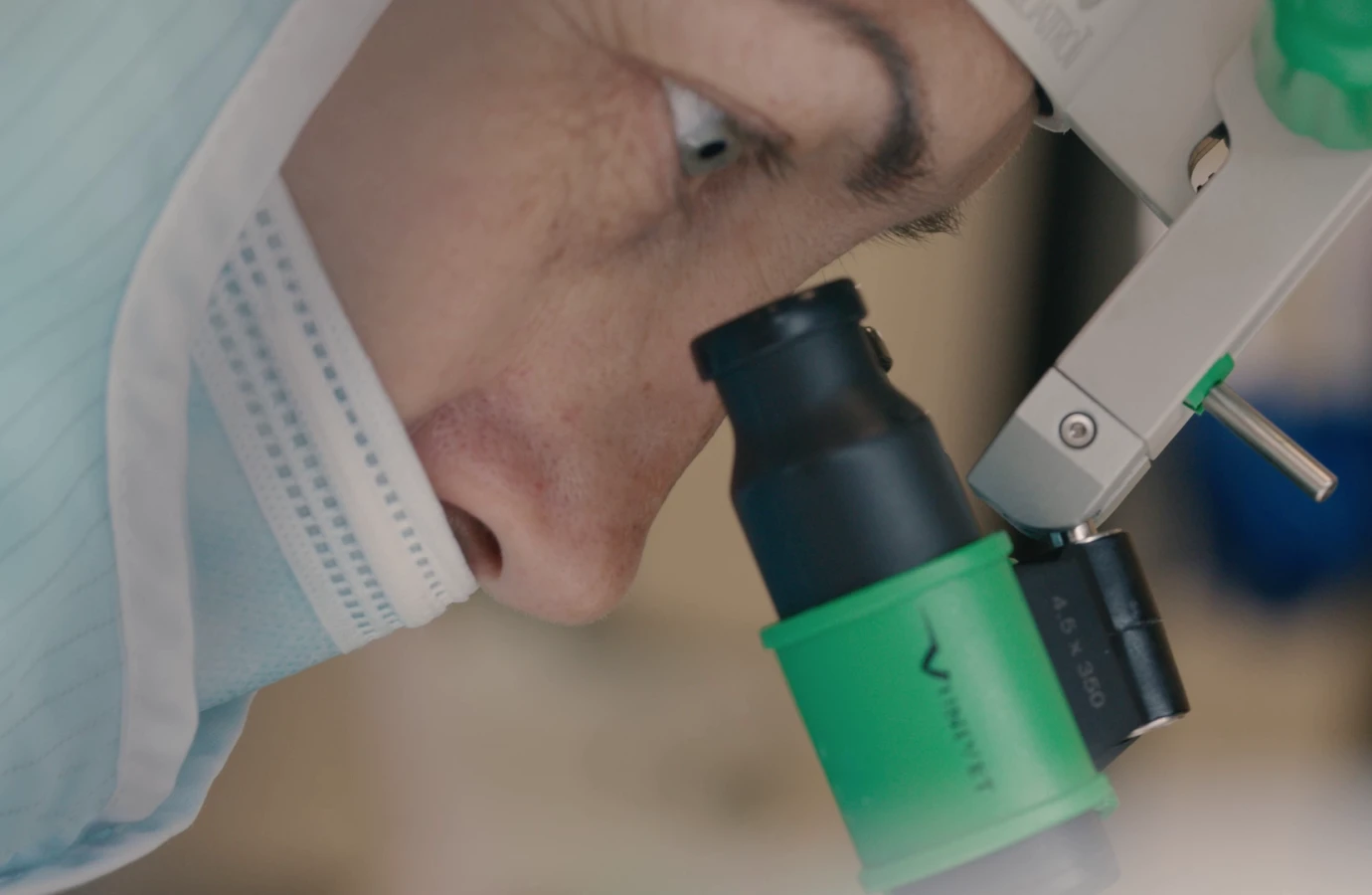
We are equipped for the production of devices of every risk class, 1, 2a, 2b, 3, and implantable, across all medical specialty areas. We guarantee the full documentary traceability of every production batch, in accordance with relevant regulations.
ENKI's flexible organizational and business model allows us to serve as a contract manufacturer for devices with both small and large production runs.
The production strategy and level of engineering are discussed and established with the client to find the best balance between investment and productivity.
Precision in assembly, pursuit of maximum repeatability, adoption of cutting-edge technological solutions, whether for large-scale or small-batch productions, is a constant objective of ENKI's engineering department.
Transparency and reliability in communication and documentation provided are fundamental to relationships that benefit all parties involved, from the client to ENKI, to the end user, and most importantly, to the ultimate beneficiary.